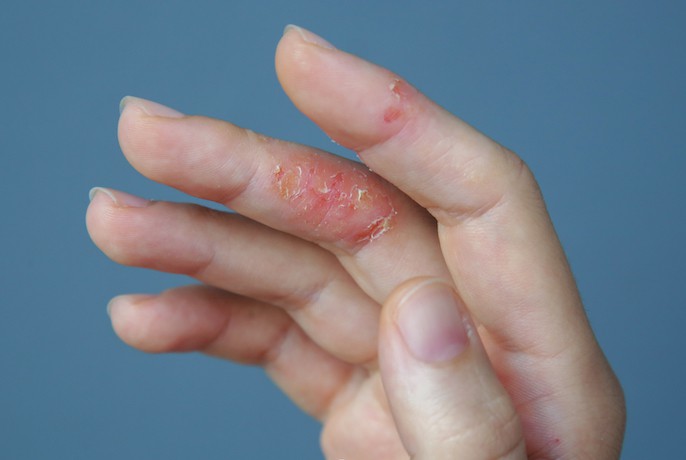
AbbVie has presented new data showing that Rinvoq (upadacitinib) monotherapy induced significant improvements in skin clearance in people with atopic dermatitis.
In the Phase III Measure Up 1 Study, of patients receiving the drug at a dose of 15mg or 30 mg, 70% and 80%, respectively, achieved a 75% improvement in the Eczema Area Severity Index (EASI 75) at week 16, respectively, versus 16% in the placebo group.
Also, 48% and 62%, respectively, achieved a validated Investigator’s Global Assessment for Atopic Dermatitis (vIGA-AD) of clear or almost clear (0/1) at week 16, versus 8% of patients receiving placebo.
For both doses, patients experienced an early reduction in itch, which was maintained through week 16. Clinically meaningful reduction in itch was defined as improvement in Worst Pruritus Numerical Rating Scale (NRS), which was achieved by a significantly higher proportion of patients receiving the drug 15mg/30 mg at week 16 compared to placebo (52%/60%, respectively, versus 12%).
Clinically meaningful reductions in itch compared to placebo were observed as early as one day after the first dose for patients receiving Rinvoq 30mg (12% versus 4%) and two days after the first dose for those on the 15mg dose (16% versus 3%).
“People with atopic dermatitis often struggle with relentless skin and itch symptoms, resulting in a significant unmet need,” said Michael Severino, vice chairman and president, AbbVie. “We’re excited by these results, which show the potential of Rinvoq for individuals living with the burden of atopic dermatitis.”
Rinvoq was approved last year on both sides of the Atlantic for rheumatoid arthritis.
Phase III trials of Rinvoq in atopic dermatitis, rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis and giant cell arteritis are ongoing.